Webinar on
Indian Regulatory Envioronment Clinical Trials and New Drug
5 September 2020
Department of Clinical Research organized a Webinar on “INDIAN REGULATORY ENVIORONMENT CLINICAL TRIALS AND NEW DRUG ”for students, research scholars, faculty members & industry processional to gain the knowledge Indian regulatory environment clinical trials and new drug on 5th September 2020. Total 72 participants were registered from various institutes, Pharmaceutical industry & Universities of the Gujarat.
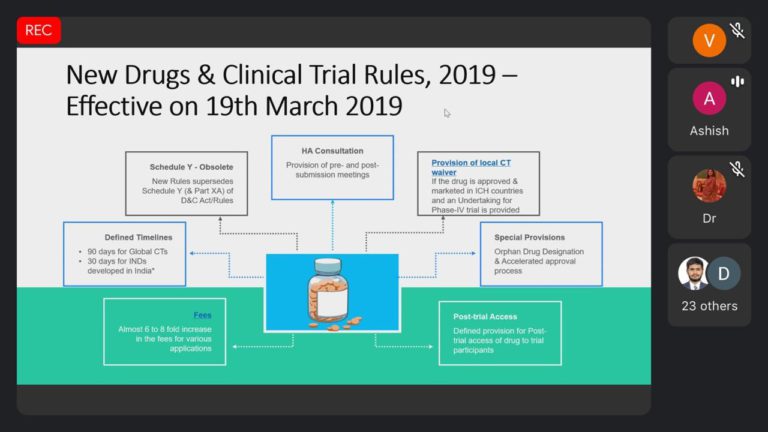
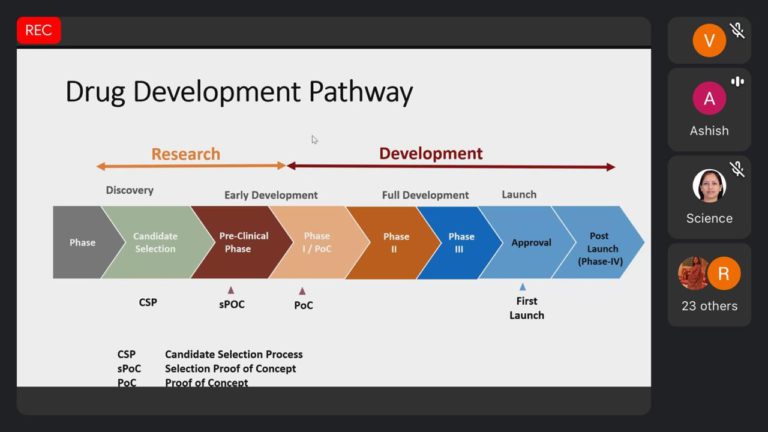
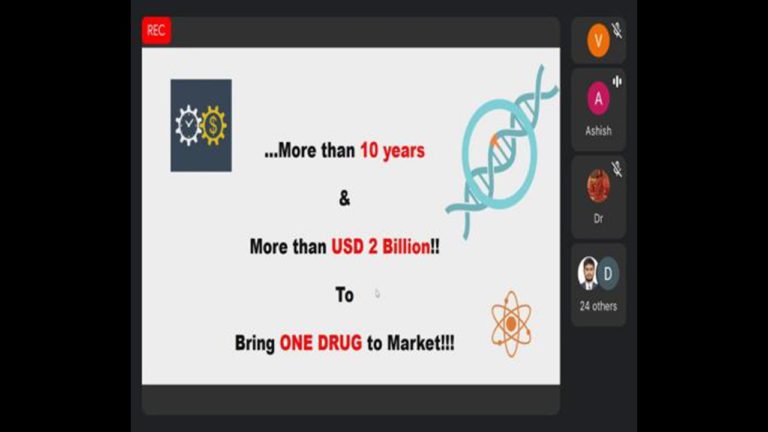
The invited speaker Mr. Ashish Chavda share Drug development process, guidelines and rules about new drugs, clinical trial new drug rules, Sugam software application,Dossier preparation, submissions and managing the operational and regulatory aspects for Global clinical trials and BA/BE studies
Presentation followed by interactive question answer session. Discussion was also on corona vaccine discussion. Participants were actively involved. The feedback received from the participants were encouraging and amazing. Audience wants more Webinar in future also.
Get in touch
For Admission Contact
Engineering +91 9909963221
Management +91 9924202777
Architecture +91 9909963221
Design +91 7622007501 / +91 8511132234
Science & Liberal Studies +91 9825865103
Computer Application +91 7622007507
Aviation +91 7227 037781
Quick Links
Contact Us
Indus University
Rancharda, Via: Shilaj,
Ahmedabad – 382 115.
Gujarat, INDIA.
Telephone: +91 2764 260277 / 78 / 79
Mobile : +91 9909963221 +918511132237
E-mail: info@indusuni.ac.in